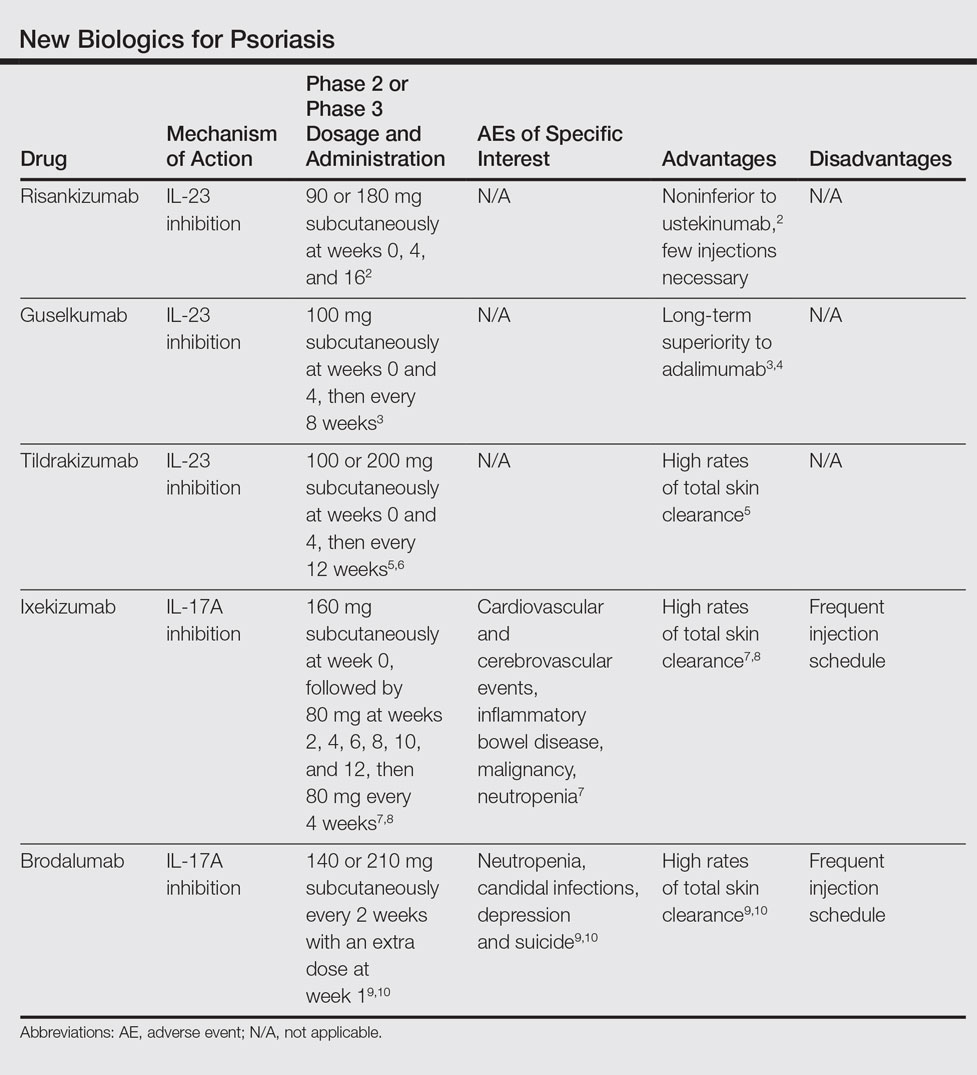
Mar 23, 18 · Ilumya marks the second interleukin23 (IL23) inhibitor to gain FDA approval within the past year, following the approval of J&J's Tremfya (guselkumab) in July 17 Share Article On March 21, the FDA approved Sun Pharma's Ilumya (tildrakizumab) for the treatment of adults with moderate to severe plaque psoriasis (PsO) who are candidatesJun 03, · Ustekinumab is a human monoclonal antibody directed against IL12 and IL23, thereby interfering with Tcell differentiation and activation and subsequent cytokine cascades ItNov 25, · Among the most recent biologic drugs available for psoriasis therapy, those targeting interleukin17 (secukinumab and ixekizumab) or its receptor (brodalumab) have been shown to be quickly effective However, in those patients who failed one or more of the abovecited drugs, reallife data on the effectiveness of switching to one anti

Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor
Role of il 17 in psoriasis
Role of il 17 in psoriasis-Interleukin23 is a heterodimeric cytokine composed of an IL12B (IL12p40) subunit (that is shared with IL12) and the IL23A (IL23p19) subunit IL23 is part of IL12 family of cytokines A functional receptor for IL23 (the IL23 receptor) has been identified and isApr 27, 21 · AbbVie's antiIL23 antibody Skyrizi and Johnson & Johnson's Tremfya and Stelara, which hits IL12 and IL23, are already approved in plaque psoriasis Despite trailing other antiIL23 drugs, Lilly moved mirikizumab into a broad phase 3 program that included three psoriasis studies, including a longterm assessment of its safety and



Deucravacitinib Miracle Drug For Autoimmune Diseases Biopharma Media
Jul 17, 19 · Plaque Psoriasis Treatments BMS Promising & Adalimumab Biosimilar Demonstrates Equivalence Published October 22, 18 KiralyLiebendorfer A The latest generation of psoriatic disease treatment National Psoriasis Foundation Published December 4, 18 Lampner C Managing Psoriatic Arthritis Updated 18 Recommendations From ACR/NPFIL23 Inhibitors Risankizumab Risankizumab (formerly known as BI ) (Boehringer Ingelheim) is a selective human monoclonal antibody Guselkumab Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL23 Tildrakizumab Tildrakizumab (formerlyAug 05, · The efficacy of interleukin (IL)23 targeted drugs for the treatment of moderate to severe psoriasis found support in results from a metaanalysis published in Dermatologic Therapy Pooled outcome data from 14 randomized clinical trials revealed that guselkumab and risankizumab were associated with the greatest improvements in psoriasis area and severity
Jul 14, · TREMFYA ® (guselkumab) Approved by US Food and Drug Administration as the First Selective Interleukin (IL)23 Inhibitor for Active Psoriatic Arthritis In two Phase 3 clinical trials, TREMFYA significantly improved signs and symptoms in joints, skin and soft tissue in adults with active psoriatic arthritisOct 09, 15 · Boehringer says antiIL23 drug beats Stelara in psoriasis trial New data from a phase II trial of Boehringer Ingelheim's psoriasis candidate BI back up earlier results showing it is more effective than a rival drug from Johnson & Johnson After nine months' treatment, 69% of moderatetosevere plaque patients treated with the highestIntroduction IL23 (InterLeukin23) produces a cutaneous phenotype in mice frequently studied as an acute model of human psoriasis IL23 induced Psoriasis Mouse Model is a convenient, easytouse and affordable mouse model of acute inflammatory response, which is widely used in mechanistic pharmacology of pathology and as a preclinical animal model for drug screening
Jun 05, 17 · Interleukin 23 inhibitors for psoriasis not just another number Major advances have been made in the past 30 years in elucidating the pathogenesis of psoriasis, including the identification of Thelper 17 (Th17) lymphocytes as key players in psoriatic inflammationBiologic agents directed towards IL23 represent new options for treatment of psoriasis 1 To date, there are not certain data on safety of biologic drugs in the treatment of pregnant women, although studies have been published Certolizumab seems to have the best safety profile on pregnantNov 12, · IL23 drugs available now for psoriasis include Guselkumab (Tremfya) Risankizumabrzaa (Skyrizi) Tildrakizumabasmn (Ilumya) New biologic treatments are in clinical trials now, including another




Applying Science To Improve The Individualized Treatment Of Patients With Psoriasis Abrar Qureshi Md Mph Chief Of The Department Of Dermatology Rhode Ppt Download




Psoriatic Arthritis Established Newer And Emerging Therapies
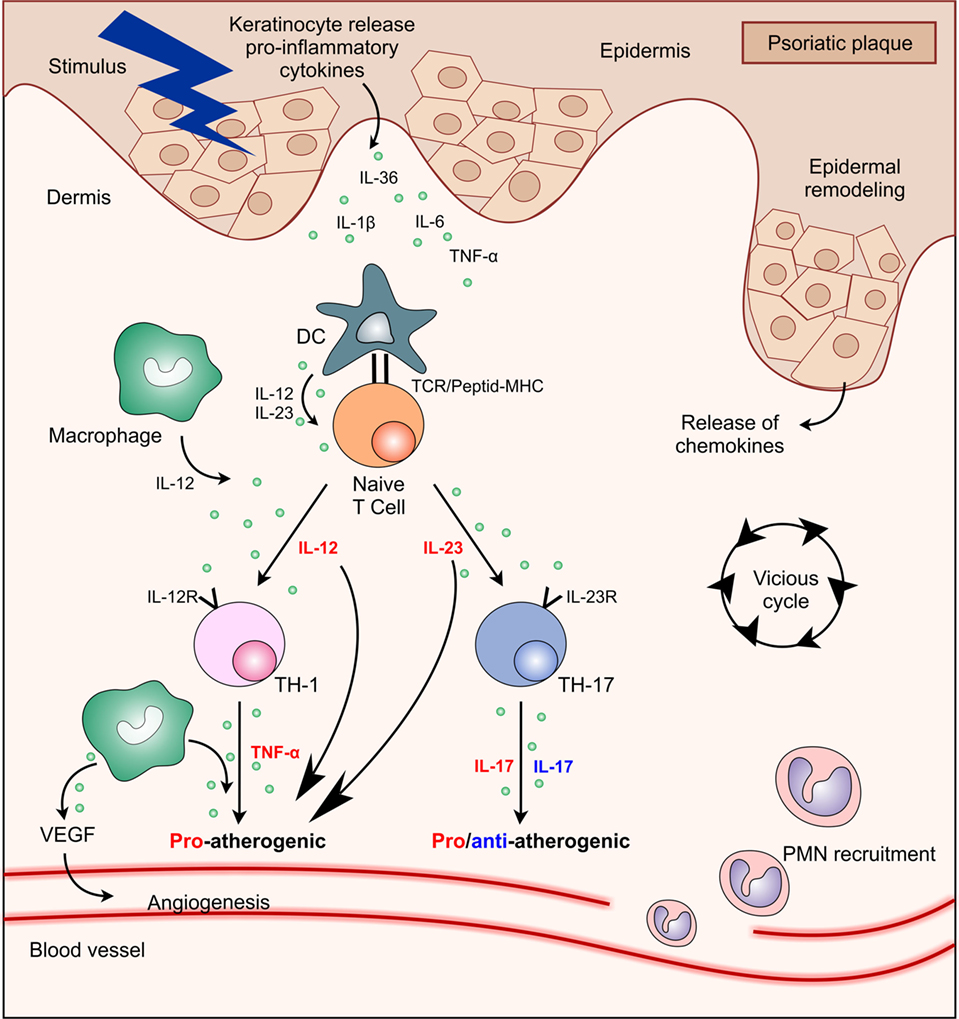
Apr 03, 21 · The balance between the key cytokine circuits in psoriasis might help to explain some of the clinical manifestations of the disease, with IL23 and IL17 dominating in plaque psoriasis, 80 interferon responses being most prominent in early, developing plaques of psoriasis, 81 and paradoxical psoriasis reactions seen in patients on antiTNFαApr 27, 21 · Eli Lilly Scraps Psoriasis Drug in Surprising Move Published April 27, 21 By Mark Terry BioSpace Eli Lilly and Company released its firstquarter 21 financial report, showing an increase in revenue of 16%, driven by 17% volume growth Even excluding the $8101 million from its COVID19 antibodies, revenue growth was 7%Sep 01, 18 · Abstract Numerous biologics are currently licensed for the treatment of psoriasis, including new drugs targeting interleukin17 (IL17) and interleukin23 (IL23) This metaanalysis evaluated the shortterm (12–16 weeks) efficacy and safety of biologics targeting IL17 and IL23 in the treatment of moderatetosevere plaque psoriasis




Deucravacitinib Miracle Drug For Autoimmune Diseases Biopharma Media



Systemic Psoriasis Therapies And Comorbid Disease In Patients With Psoriasis A Review Of Potential Risks And Benefits Jcad The Journal Of Clinical And Aesthetic Dermatology
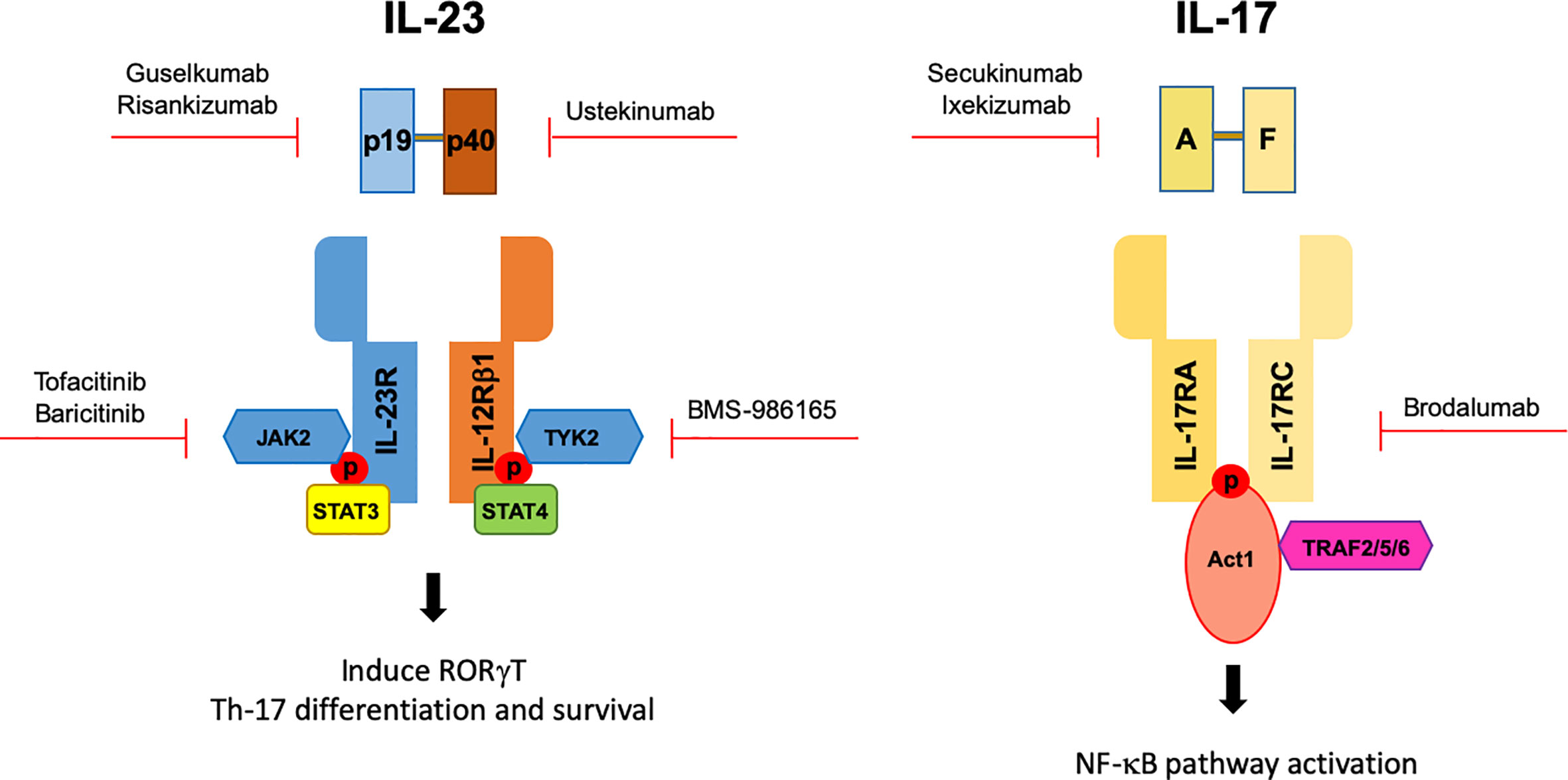
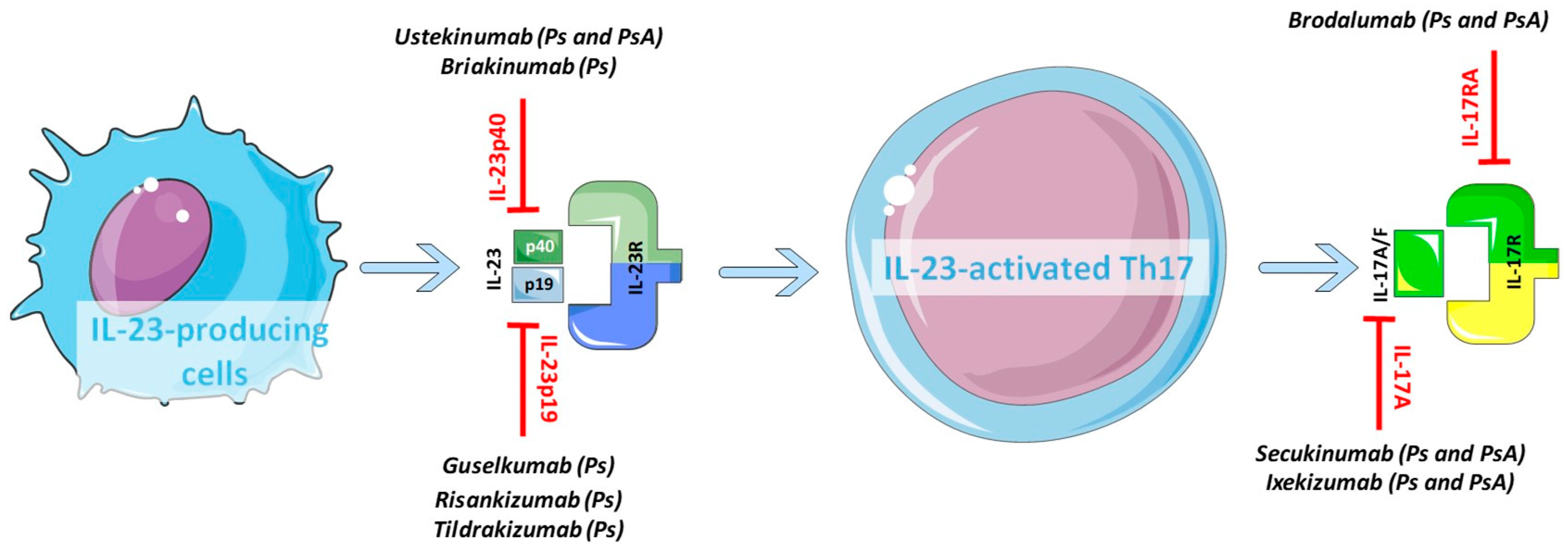
Apr 29, · Therapies targeting either IL23 or IL17 have shown great efficacy in psoriasis and have helped augment our understanding of psoriasis pathogenesis Therapies such as ustekinumab and guselkumab inhibit IL23 Ustekinumab targets the p40 subunit common to both IL23 and IL12 while guselkumab targets the p19 subunit found in IL23Jun 16, 21 · Interleukin 12 and 23 (IL12 and IL23) inhibitor Stelara (ustekinumab) selectively targets IL12 and IL23, both of which are involved in the inflammation cascade that leads to psoriasis It isAbstract Since the identification of high levels of interleukin 23 (IL 23) in psoriasis lesional skin, as well as finding that IL23 was the most important source of the p40 subunit shared by IL12 and IL23, significant effort has been made in identifying potential new drugs that specifically block the unique IL23 p19 subunit At this time, 2 inhibitors of IL23 p19 have been approved by the



A Closer Look At Skyrizi Practical Dermatology




Spotlight On Risankizumab And Its Potential In The Treatment Of Plaque Ptt
IL17 and IL23 inhibitors are the most recently approved biologic agents for psoriasis and work by blocking different molecules called interleukins, which are inflammatory signals Ustekinumab and secukinumab target IL12/23 and IL17 receptor, respectively The newer IL23 specific inhibitors include guselkumab and tildrakizumabIt is a biologic agent similar to guselkumab and tildrakizumab which targets IL23 specifically, and has been primarily developed for use in moderatetosevere psoriasis USAbased pharmaceutical company Abbvie submitted it for a Biologics License Application to the US Food and Drug Administration (FDA) in April 18Mar 22, 18 · Meanwhile, AbbVie has an IL23 blocker called risankizumab in latestage testing that it thinks could be a bestinclass treatment and a $1bnplus contender in psoriasis With competition heating up particularly in patients who have already been treated with TNF blockers analysts have predicted that Ilumya is likely to be a modest product




Emerging Landscape In Psoriasis Management From Topical Application To Targeting Biomolecules Sciencedirect




Pathophysiology And Inhibition Of Il 23 Signaling In Psoriatic Arthritis A Molecular Insight Sciencedirect
Jan 21, 21 · In another network metaanalysis study including 77 trials (34,816 patients), investigators assessed the relative efficacy of IL17– and IL23–targeted treatments for moderate to severe psoriasis, comparing the shortterm efficacy of available (or imminently available) biologic and nonbiologic systemic therapies, reflected in the PASI responseFeb 10, · There are a number of new psoriasis treatments for people with moderate to severe psoriasis Learn about biologics like IL17 inhibitors and IL23 inhibitors Discover emerging oral and topicalMar 23, 21 · "The IL23 drugs target the p19 gene which has been directly linked to psoriasis and that may give them a competitive advantage over the IL17 and TNF biologics that work through other pathways" But the IL17 inhibitors have surpassed the IL23 inhibitors in a different way




Il 23 Inhibition In Psoriasis A Novel Approach To Convenient Consistent Clearance European Medical Journal




Redoxis Models Of Psoriasis And Psoriatic Arthritis
Aug 06, · Guselkumab is a fully human monoclonal antibody that binds to the p19 subunit of IL23 9 Guselkumab was the first IL23 antagonist to receive FDA approval for the treatment of moderate to severe plaque psoriasis in adults who are candidates for phototherapy or systemic therapy The monoclonal antibody was approved in 17 based on results from 2 doubleblind, placebocontrolled clinical trials that included participants with moderate to severe psoriasisOct 09, 18 · Tremfya (guselkumab) is given once every two months and it's the first to be approved that selectively blocks a protein known a cytokine IL23 p19 Another drug called Ilumya (tildrakizumab) is given every three months and was approved only last monthMar 13, · Types of IL23 inhibitor Guselkumab (Tremfya) Risankizumabrzaa (Skyrizi) Tildrakizumabasmn (Ilumya)




Fda Approves Guselkumab For Psoriatic Arthritis



Lirpos Urac 30 Morocco Psoriatic Arthritis Management And
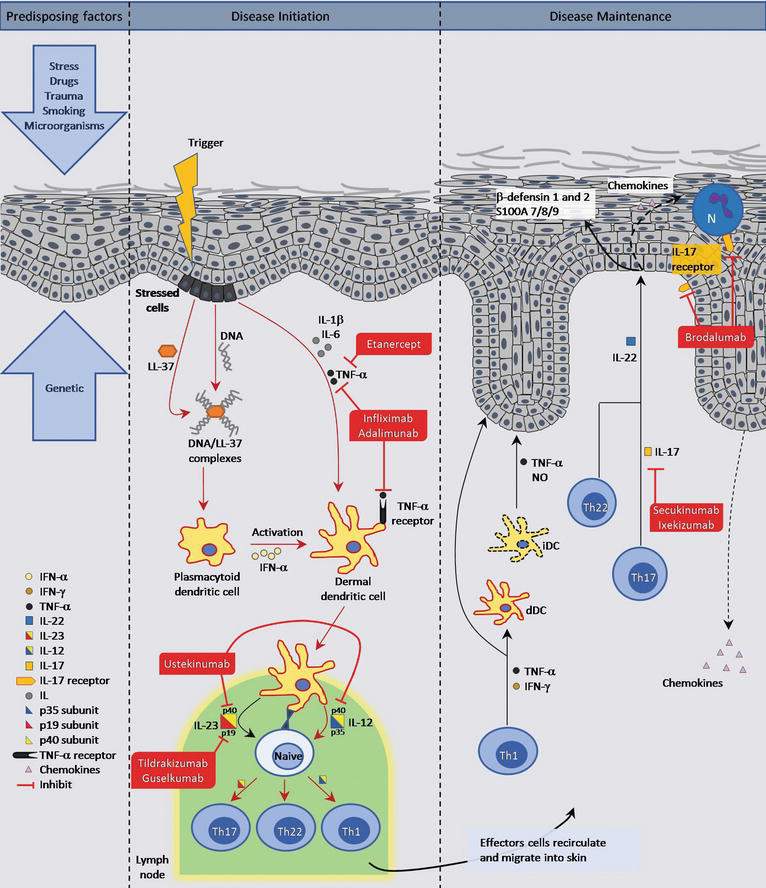
Dec 15, · While these drugs are very effective, drugs that block IL23 are among the most promising psoriasis treatments 1,3,7 Many genes associated with psoriasis correspond to the genes for the two subunits of IL23 (p40 and p19) and the genes for the IL23 receptor 1 Drugs that block IL23 require few injections (as little as every 3 months), areJan 29, 21 · Psoriasis is an immunemediated inflammatory skin disease associated with numerous inflammatory comorbidities, including increased cardiovascular risk The interleukin (IL)23/IL17 axis plays a central role in the immunopathogenesis of psoriasis andHowever, these conventional treatments may not be sufficiently effective and are associated with numerous side effects 2 In recent years, the advent of biological therapy provides new therapeutic options for psoriasis, including interleukin (IL)12/23, IL17, and IL23 inhibitors In particular, IL23 is the latest cytokine discovered to play




Il 23 Inhibitors And Other Emerging Psoriasis Treatments Youtube




Tildrakizumab Monoclonal Antibody Drug Used In Treatment Of Psoriasis Targets Interleukin 23 Generic Name And Stylized Antibody Stock Illustration Illustration Of Nomenclature Biosimilar
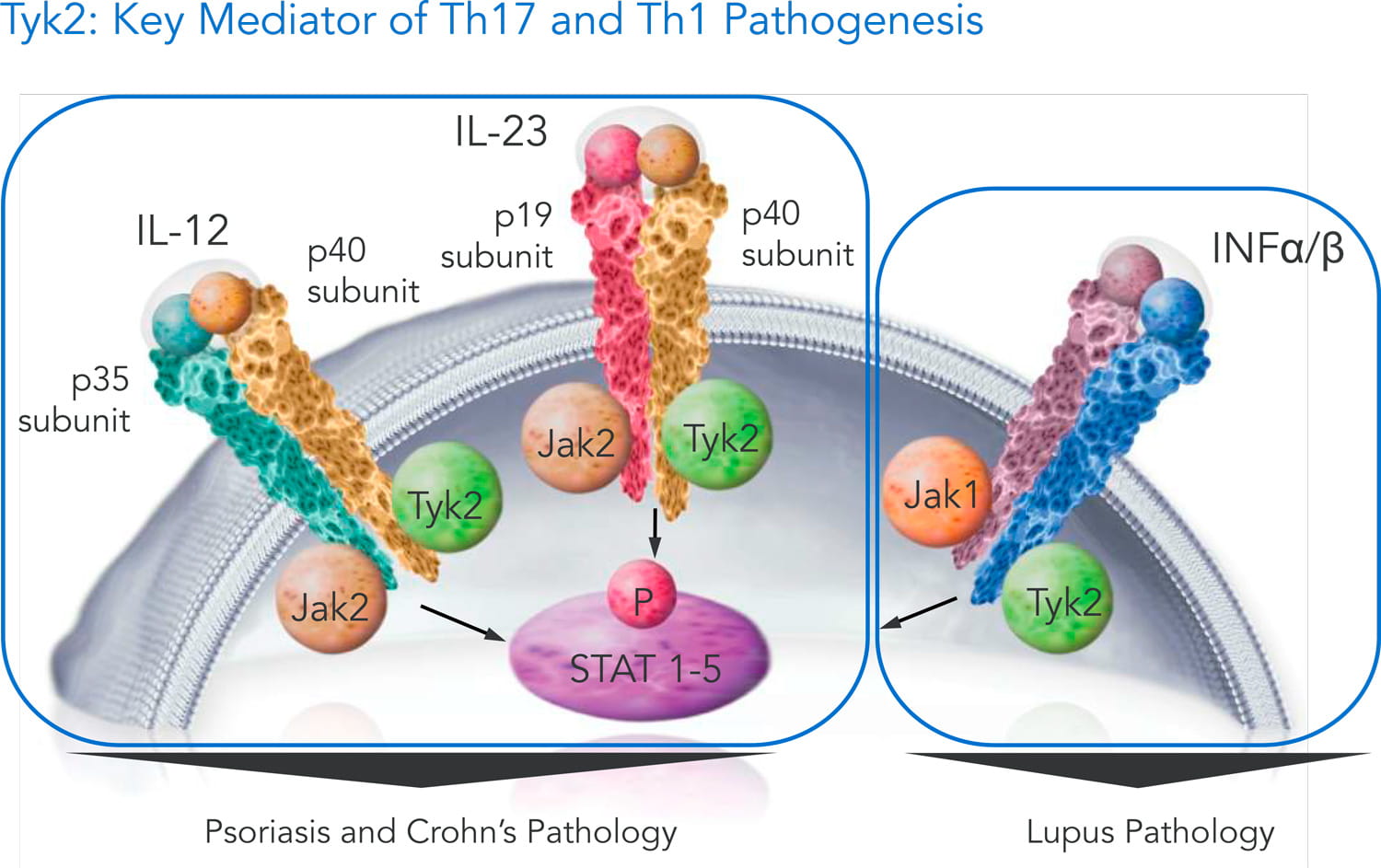
Mar 31, · Get the facts on nearly new and available psoriasis treatments for adults and kids Also learn about others that are on the horizon The drug works by blocking IL23Mar 01, 18 · Several IL23 inhibitors are in clinical development, including guselkumab It is the first IL23 inhibitor to be approved for the treatment of patients with moderatetosevere plaque psoriasis in the USA and is in Phase II evaluation for use in psoriatic arthritisJul 06, · TYK2 is an intracellular signaling kinase (enzyme) As with the other medications, the goal is to stop the immune and proinflammatory signaling pathway that leads to chronic inflammation and diseases such as psoriasis TYK2 mediates signaling of IL23, IL12, and type I interferondriven responses




Rig I Antiviral Signaling Drives Interleukin 23 Production And Psoriasis Like Skin Disease Embo Molecular Medicine




Figure 1 From Pathogenic Role Of Il 17 In Psoriasis And Psoriatic Arthritis Semantic Scholar
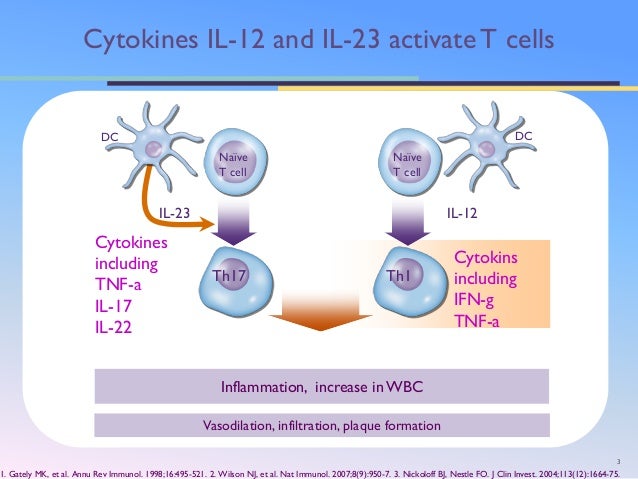
Human IL23 is primarily produced by antigenpresenting cells and induces and maintains differentiation of Th17 cells and Th22 cells, a primary cellular source of proinflammatory cytokines such as IL17 and The role of IL 23 in the treatment of psoriasis Expert Rev Clin ImmunolApr 27, 21 · AbbVie's antiIL23 antibody Skyrizi and Johnson & Johnson's Tremfya and Stelara, which hits IL12 and IL23, are already approved in plaque psoriasis Despite trailing other antiIL23 drugsFour effective antiIL 23 drugs for psoriasis Two approved and two pending antiIL 23 drugs offer an opportunity for moderate to severe psoriasis patients to regain their lives, according to researchers presenting at the 18 Fall Clinical Dermatology Conference




Mechanism Of Action Tremfya Guselkumab Hcp




Psoriasis Treatment Unmet Needs Present 19 Opportunities Pm360
Mar 04, · "The key molecule in treating psoriasis is blocking IL17 What IL23 does is it downregulates the TH17 cells production of IL17," Mark Lebwohl, MD, said during the presentation "When youMar 08, 21 · IL23 is an important cytokine involved in the pathogenesis of psoriasis;Oct 03, 16 · The trouble, though, is that their drug was clearly overshadowed by guselkumab, another IL23 drug that's now angling to hit the market




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology




Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor
Jul 17, · Eli Lilly's antiIL23 antibody beat placebo at clearing psoriasis symptoms in a phase 3 study, teeing up regulatory filings across the globe But that's not all—the drug also outshone




The Role Of Il 17a In Axial Spondyloarthritis And Psoriatic Arthritis Recent Advances And Controversies Annals Of The Rheumatic Diseases




Interleukin 23 And Interleukin 17 Importance In Pathogenesis And Therapy Of Psoriasis




Interleukin 23 Inhibitors For Psoriasis Not Just Another Number The Lancet
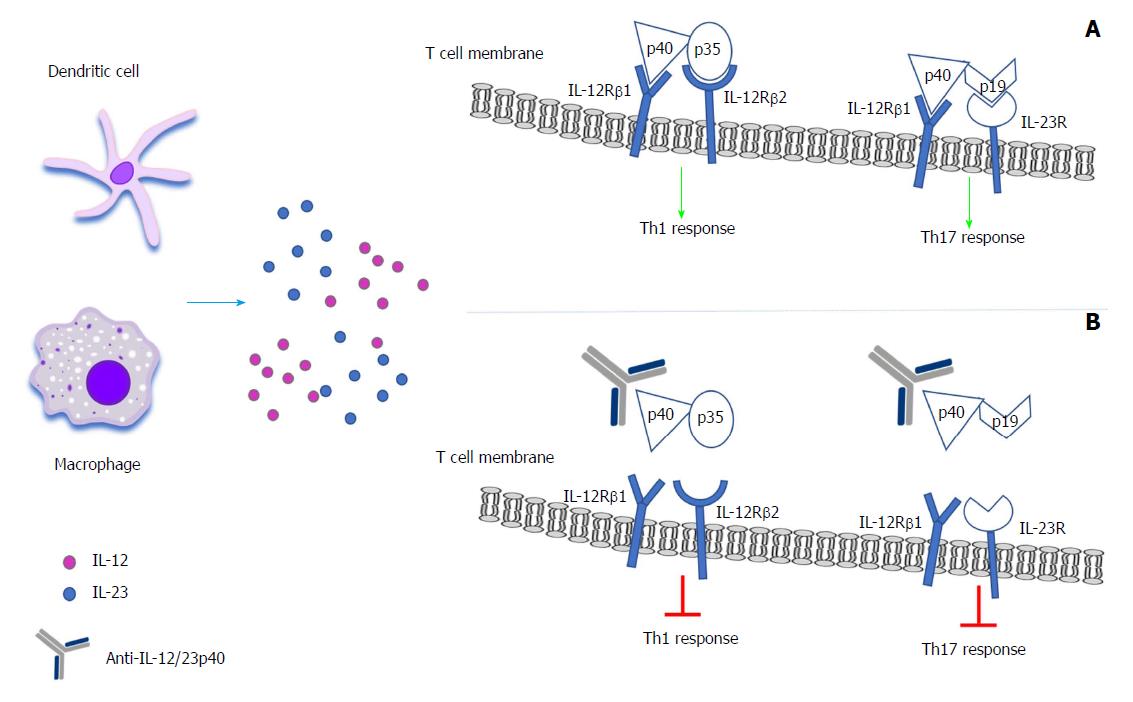



Interleukin 12 Interleukin 23 Pathway Biological Basis And Therapeutic Effect In Patients With Crohn S Disease




Guselkumab Monoclonal Antibody Drug Targets Il 23 Subunit Alpha Used In The Treatment Of Psoriasis Generic Name And Stylized Antibody Representatio Stock Photo Alamy




Mechanism Of Action Moa Ilumya Tildrakizumab Asmn Hcp




Current Model Of The Pathophysiology Of Psoriasis Il 23 Bridges The Download Scientific Diagram




Current Developments In The Immunology Of Psoriasis Abstract Europe Pmc



Il 23 Induces Atopic Dermatitis Like Inflammation Instead Of Psoriasis Like Inflammation In Ccr2 Deficient Mice




Lilly Scraps Il 23 Psoriasis Program Despite Phase 3 Success Focuses On Ibd Race Against Abbvie J J Fiercebiotech




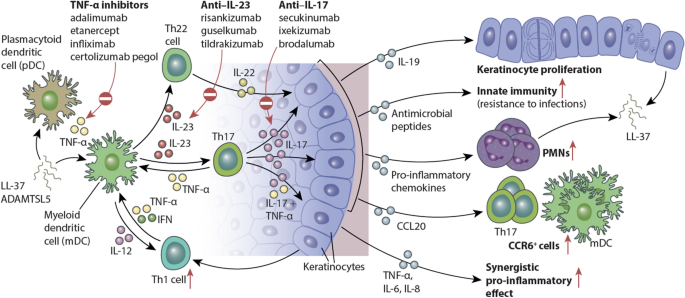
Biological Drugs Targeting Il 17 Or Il 23 Approved For Psoriasis Psa Download Scientific Diagram




Guselkumab Monoclonal Antibody Drug Targets Il 23 Subunit Alpha Stock Photo Picture And Royalty Free Image Image
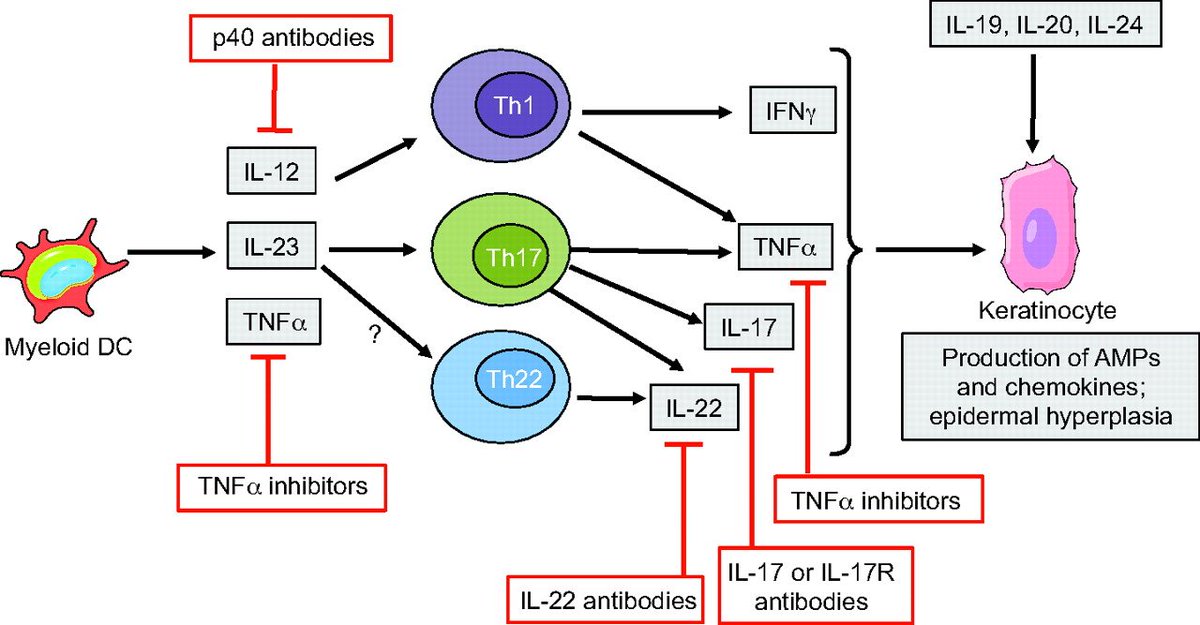



Dominique Du Crest More About Anti Interleukin 23 Il23 Drug In The Treatment Of Plaque Psoriasis Interview Of Prof Chris Griffiths T Co Fcxhb9uocj Guselkumab Dermatology Dermatologia Skinhealth T Co Qjielmab6u
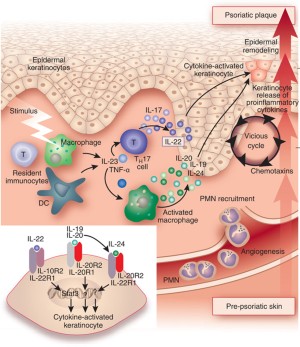



New Anti Il 23 Drugs Raise Hopes For Psoriasis Plaque Clearance Nature Biotechnology




Therapeutics Targeting The Il 23 And Il 17 Pathway In Psoriasis The Lancet



Injections To Treat Psoriasis Types Benefits And Risks




Psoriasis And Treatment Past Present And Future Aspects Html Acta Dermato Venereologica




Interleukin 23 In Psoriasis Integrating New Therapies In The Current Treatment Landscape European Medical Journal




Targeting Interleukin 23 In The Treatment Of Noninfectious Uveitis Ophthalmology




Fda Approves Tremfya First Il 23 Inhibitor For Psoriatic Arthritis




Abbvie S Skyrizi Stands To Gain As Docs Move Away From Older Psoriasis Meds Amgen S Otezla Included Fiercepharma



Deucravacitinib Miracle Drug For Autoimmune Diseases Biopharma Media




Plaque Psoriasis Drug Shows Promise For Psoriatic Arthritis Medpage Today




Management Of Plaque Psoriasis A Review And Comparison Of Il 23 Inhibitors European Medical Journal




6 Key Domains Of Psoriatic Arthritis Manifestations Otezla Apremilast Information For Global Healthcare Professionals
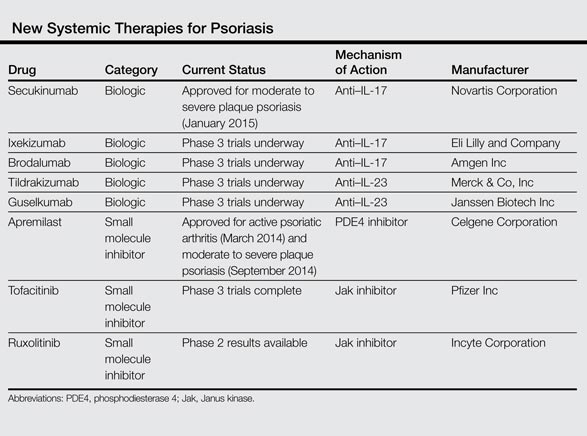



New Systemic Therapies For Psoriasis Mdedge Dermatology
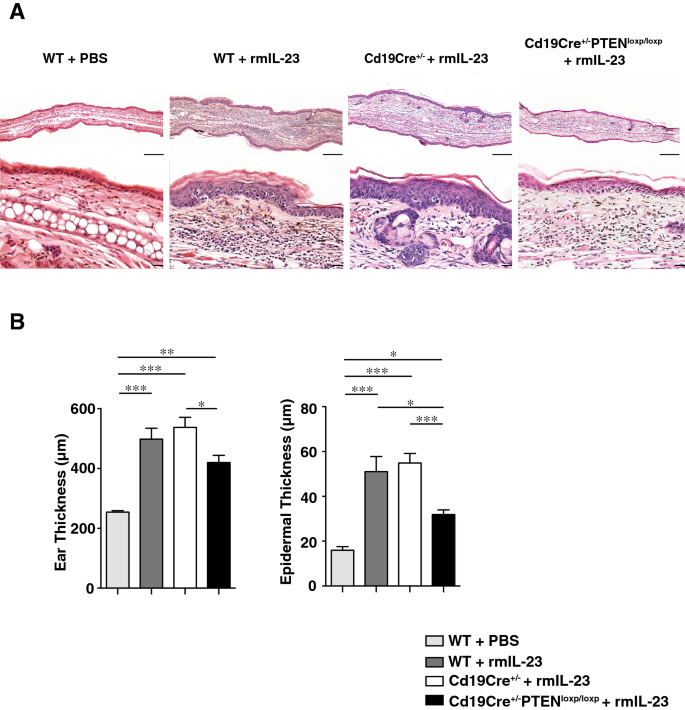



Suppression Of Il 23 Mediated Psoriasis Like Inflammation By Regulatory B Cells Scientific Reports




Cell Line Of The Month Il 23




New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram
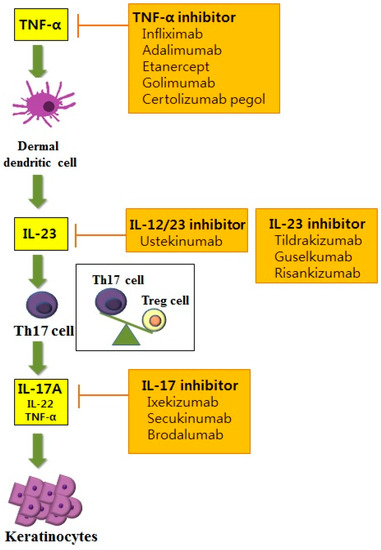



Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html




New Treatments For Atopic Dermatitis Targeting Beyond Il 4 Il 13 Cytokines Annals Of Allergy Asthma Immunology



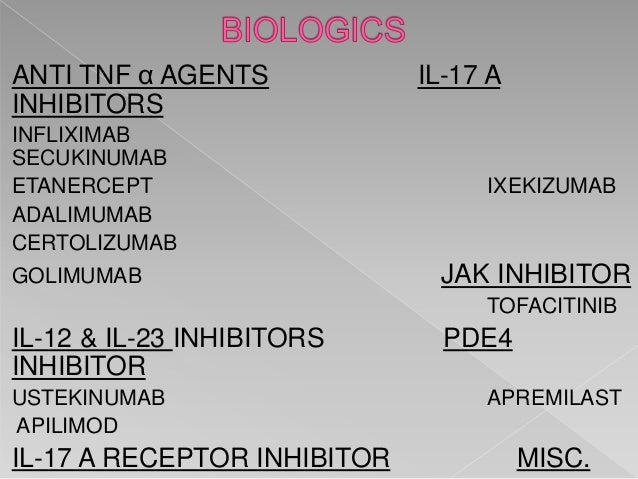
Full Article Monoclonal Antibodies Inhibiting Il 12 23 And 17 For The Treatment Of Psoriasis



New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology




Biologic Treatments Of Psoriasis An Update For The Clinician Btt




Pharmacist Medication Insights Risankizumab Rzaa For Moderate To Severe Plaque Psoriasis




The Role Of Il 17a In Axial Spondyloarthritis And Psoriatic Arthritis Recent Advances And Controversies Annals Of The Rheumatic Diseases



Ustekinumab Therapy Efficacy In Psoriasis Patients Previously Treated




Top Pdf Anti Il 23 Antibody 1library




The Il 23 Th17 Axis In The Immunopathogenesis Of Psoriasis Sciencedirect




Psoriasis Nejm




Rig I Antiviral Signaling Drives Interleukin 23 Production And Psoriasis Like Skin Disease Embo Molecular Medicine




Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor




Psoriasis Drugs Currently Under Development Download Table




T5z3kbruuxyafm




Il 23 Inhibitors For Treating Psoriasis What To Know



Interleukin 17 And Interleukin 23 A Narrative Review Of Mechanisms Of Action In Psoriasis And Associated Comorbidities Springerlink



Frontiers Psoriasis Cardiovascular Events And Biologics Lights And Shadows Immunology




Tremfya Vs Cosentyx Will Il 23s Surpass Il 17s In The Psoriasis Market Clinical Trials Arena




Tildrakizumab Monoclonal Antibody Drug Used In Treatment Of Psoriasis Targets Interleukin 23 Generic Name And Stylized Antibody Stock Illustration Illustration Of Immunology Monoclonal



Frontiers The Il 17 Il 23 Axis And Its Genetic Contribution To Psoriatic Arthritis Immunology




Management Of Pediatric Plaque Psoriasis Using Biologics Journal Of The American Academy Of Dermatology




Targeting Of Interleukin 17 In The Treatment Of Psoriasis Ccid




European Commission Approves Janssen S Tremfya Guselkumab A First In Class Treatment For Active Psoriatic Arthritis Psa Neuro Central



Psoriasis Recent Advances And Existing Therapy In Psoriasis



Pathogenic Role Of Cytokines And Effect Of Their Inhibition In Psoriasis Intechopen




Phase 3 Tremfya Results Are Promising For Plaque Psoriasis And Psoriatic Arthritis Drug Discovery And Development




The Evolving Landscape Of Psoriasis Treatment New Treatment Paradigms In Psoriasis Understanding And Incorporating Recent And Emerging Trends Global Academy For Medical Education



Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html
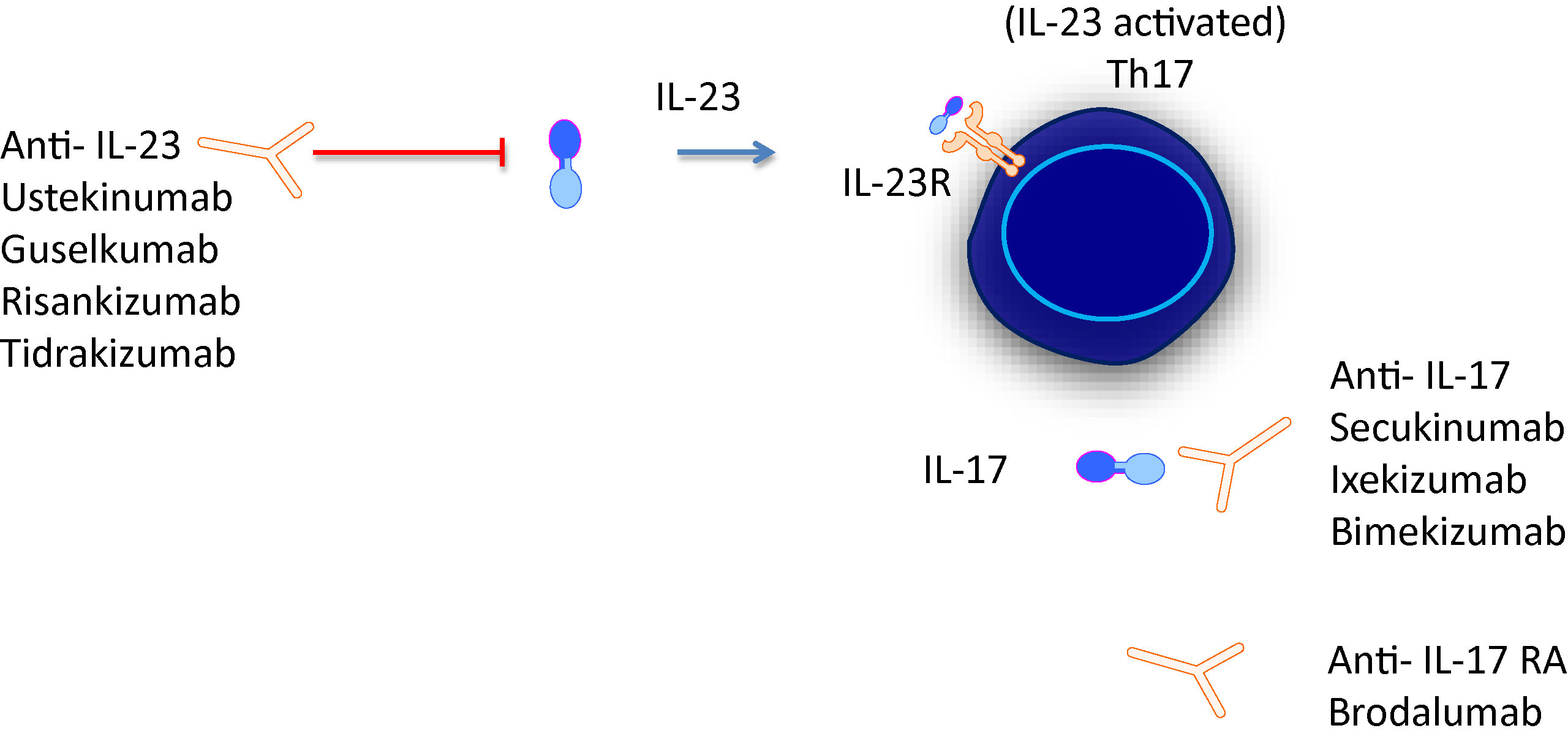



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology




Euroguiderm Guideline On The Systemic Treatment Of Psoriasis Vulgaris Part 1 Treatment And Monitoring Recommendations Nast Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library
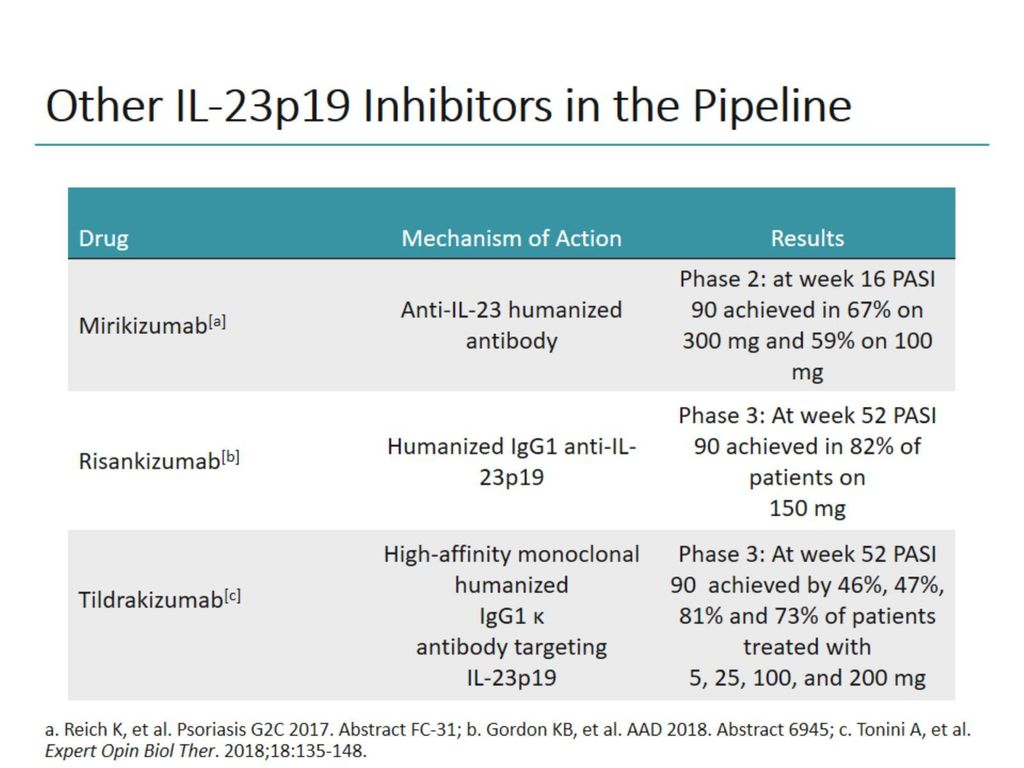



Evolution Of Treatment Strategies Targeting Il 23 For Psoriasis Ppt Download




Psoriasis Il 23 Biologic Maintains Efficacy In 2 Year Trial Medpage Today
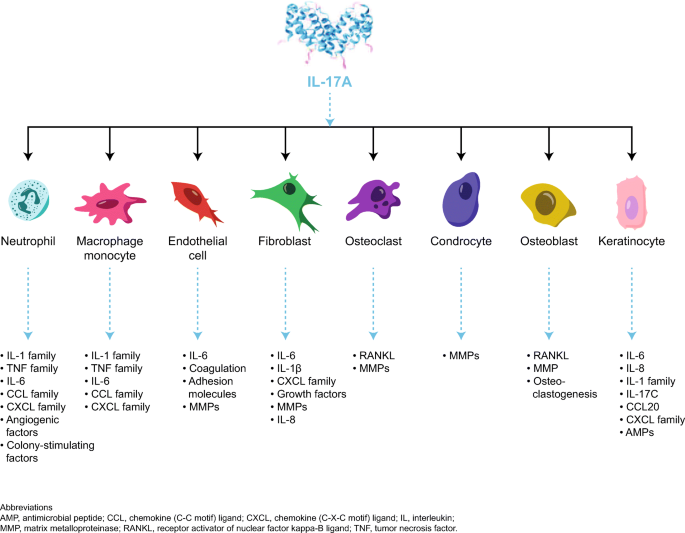



The Immunologic Role Of Il 17 In Psoriasis And Psoriatic Arthritis Pathogenesis Springerlink




Psoriasis Translating Current And Emerging Therapies Into Enhanced Management Strategies Youtube




Article Jddonline Journal Of Drugs In Dermatology




Interleukin 17 Wikipedia
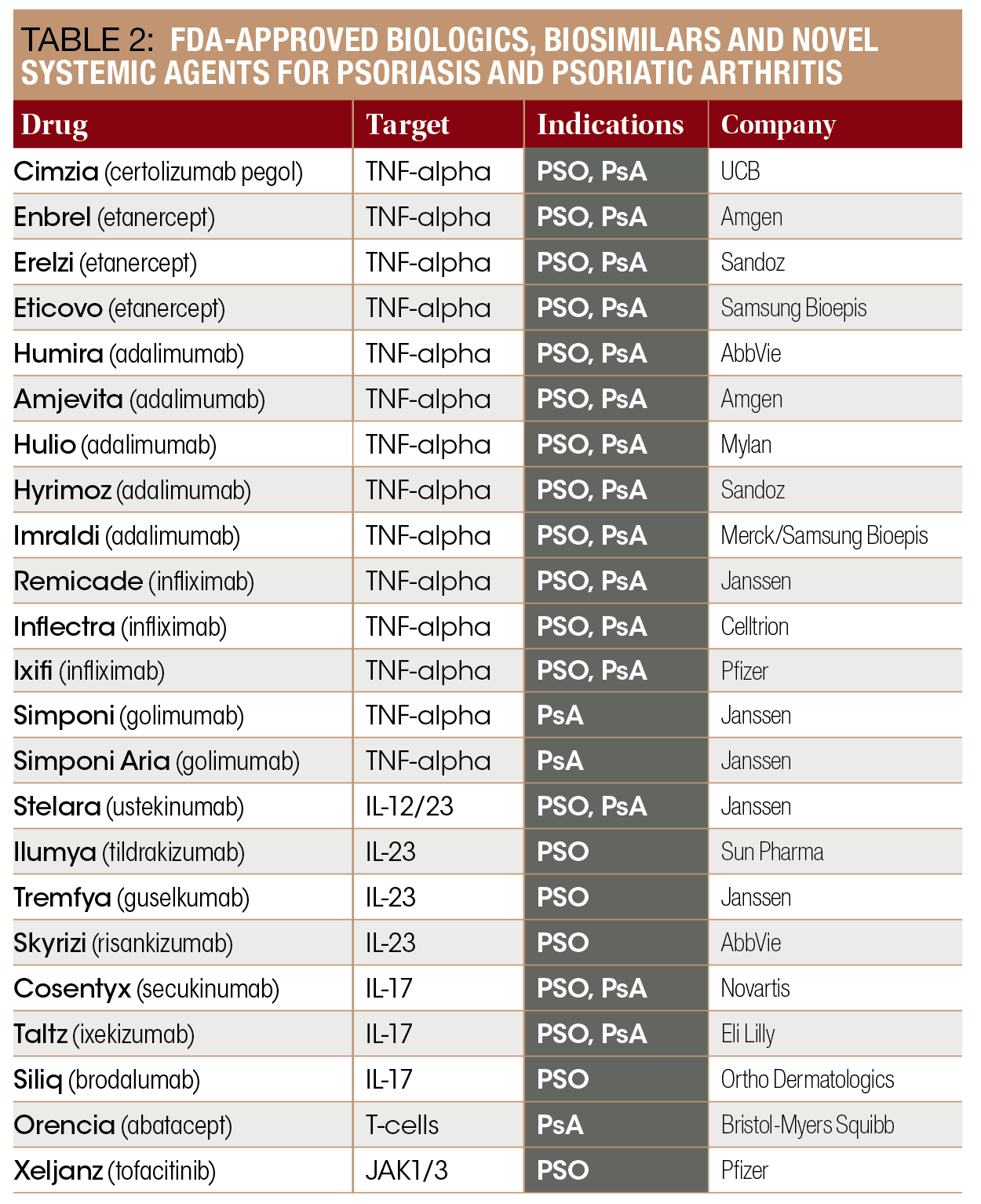



Biologics Make Psoriasis Clearance A Real Possibility




Lilly S Il 23 Drug Beats Novartis Cosentyx In Plaque Psoriasis Fiercebiotech




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology
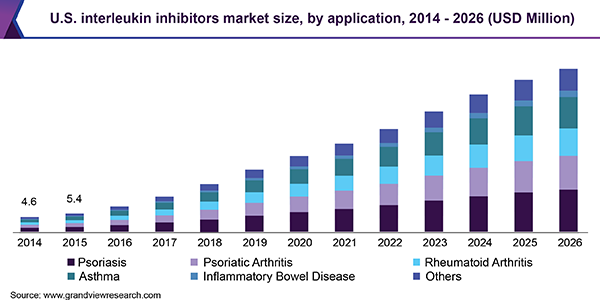



Il Inhibitors Market Size Share Industry Trends Report 19 26




The Role Of Il 23 And The Il 23 Th17 Immune Axis In The Pathogenesis And Treatment Of Psoriasis Girolomoni 17 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library




Ustekinumab And Anti Interleukin 23 Agents In Crohn S Disease Gastroenterology Clinics




May 08 International Psoriasis Council
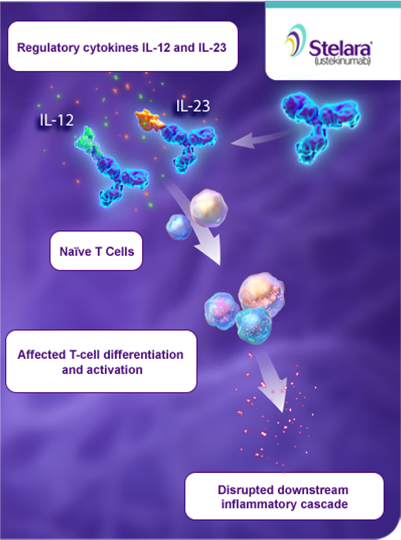



Stelara Ustekinumab Mechanism Of Action Plaque Psoriasis
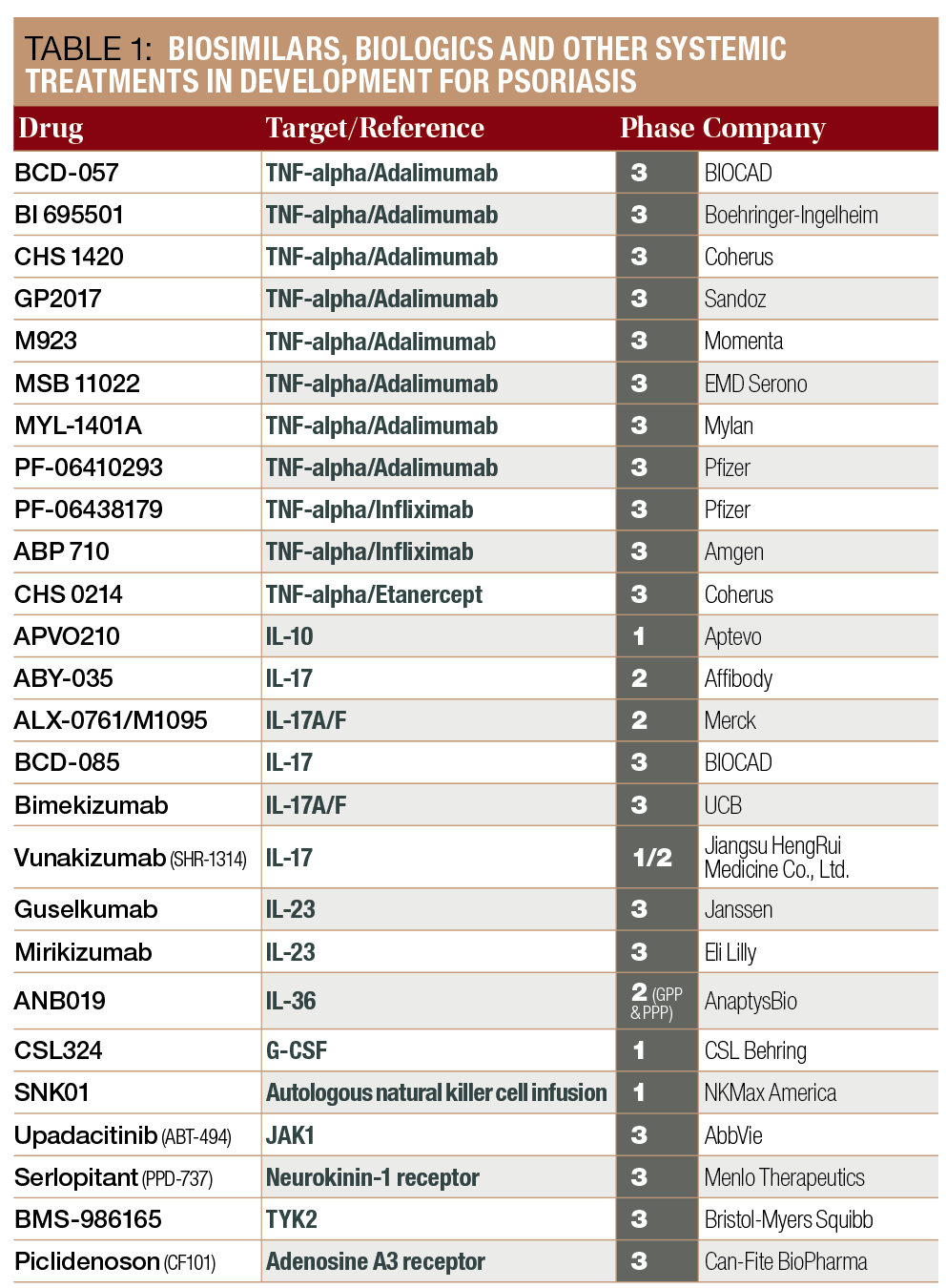



Biologics Make Psoriasis Clearance A Real Possibility




Biologicals In The Treatment Of Psoriasis The Indian Perspective Janagond Ab Palit A Blde Univ J Health Sci




The Next Wave Of Psoriasis Drug Programs Targeting Il 17 And Il 23 Pamela Spicer Analyst Cns Autoimmune Inflammation Ophthalmology Pdf Free Download




Interleukin 23 In The Skin Role In Psoriasis Pathogenesis And Selective Interleukin 23 Blockade As Treatment Semantic Scholar



Il 23 Induces Atopic Dermatitis Like Inflammation Instead Of Psoriasis Like Inflammation In Ccr2 Deficient Mice



Biologics In Psoriasis The Next Generation Practical Dermatology



0 件のコメント:
コメントを投稿